Biologics for COPD are targeted immune treatments that can reduce flare-ups in a specific subgroup of people with COPD, especially those with evidence of type 2 inflammation.
What are biologics for COPD?
Biologics are usually monoclonal antibodies. They block precise inflammatory signals rather than broadly damping the immune system. You take them by injection, most often every 2–4 weeks, alongside your usual inhalers.
In COPD, the aim is simple: reduce exacerbations (flare-ups). A flare-up can mean worsening of the condition requiring antibiotics, steroid tablets, A&E visits, or hospital admission.
Why biologics only suit some people with COPD
COPD is not one single condition. It is a label that includes different “treatable traits”. Some people have more emphysema. Others have chronic bronchitis (cough and phlegm most days). Some flare because infections drive their disease. Others flare because a particular inflammation pattern keeps switching on.
Biologics focus on one pattern that looks like “type 2 inflammation”. This is more familiar in asthma, but it also appears in a meaningful subgroup of people with COPD.
Type 2 inflammation in COPD
Blood eosinophil count is a practical marker of type 2 inflammation. It is not perfect, and it can vary, but it helps identify people who may respond to specific anti-inflammatory options. Type 2 inflammation can result in chronic bronchitis symptoms, repeated exacerbations, and ongoing symptoms despite maximal inhaler therapy.
Many of the key COPD biologic trials enrolled people with higher eosinophils (≥300 cells/µL).
Although targetting type 2 inflammation in COPD can reduce exacerbation frequency it is important to note that it does not reverse COPD damage, and that it will not suit everyone.
Which biologics have the strongest evidence in COPD?
Dupilumab (blocks IL-4 and IL-13 signalling)
Dupilumab has the most consistent phase 3 evidence in COPD so far (N Engl J Med. 2024;390(24):2274–2283). Large trials in people with COPD and raised eosinophils who still had exacerbations despite triple inhaler therapy showed fewer moderate or severe exacerbations compared with placebo. These studies also showed modest improvements in lung function.
What this means is that if you are the right candidate, you are more likely to have fewer flare-ups across the year, and you may notice modest breathing “headroom”.
Mepolizumab (targets IL-5)
Mepolizumab reduces eosinophil-driven inflammation by blocking IL-5. In a major phase 3 trial, people with eosinophilic COPD on background triple inhaler therapy had a lower annualised rate of moderate or severe exacerbations with mepolizumab than with placebo (N Engl J Med. 2025;392(17):1710–1720).
Tezepelumab (blocks TSLP)
Tezepelumab targets an “upstream” signal (TSLP) that can switch on multiple inflammatory pathways. In COPD, results have been mixed. A large study did not show a clear universal benefit across the whole COPD population, which suggests researchers still need to define which subgroup might benefit most (Lancet Respir Med. 2025;13(1):47–58).
Benralizumab (targets the IL-5 receptor on eosinophils)
Earlier large trials of benralizumab in stable COPD did not show a lower annualised exacerbation rate overall compared with placebo (N Engl J Med. 2019;381(11):1023–1034). This does not rule out future niche uses, but it means it is not currently a standard long-term biologic option for COPD in most pathways.
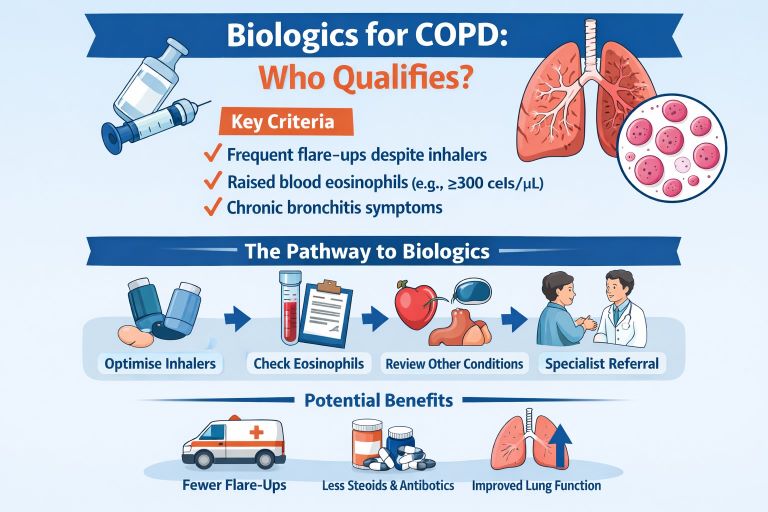
Who might be eligible for biologics for COPD in everyday care?
Most pathways follow a common logic. You are more likely to be considered if:
You have confirmed COPD and you still have exacerbations despite optimised inhaler therapy (often triple therapy).
You have raised blood eosinophils, ideally shown on more than one test.
You have ongoing symptoms that affect daily life, often with chronic bronchitis features.
Your clinician has checked and treated common “exacerbation amplifiers” such as bronchiectasis, reflux, heart failure, obstructive sleep apnoea, rhinitis, anxiety, and poor inhaler technique.
You have tackled the foundations: smoking cessation support, pulmonary rehabilitation, and vaccinations.
Where do biologics sit in the 2026 GOLD approach?
Recent global COPD guidance places greater emphasis on preventing exacerbations early and stepping up treatment when people continue to flare. Biologics now sit more clearly as an option for people who remain uncontrolled despite triple inhaled therapy and who match the phenotype studied in trials, particularly those with higher eosinophils.
What’s happening in the UK in 2026?
In the UK, biologics for COPD are expected to be delivered through specialist respiratory NHS services, with eligibility typically based on exacerbation frequency, current inhaler treatment, eosinophil counts, and symptoms.
What benefits should you expect (and what not to expect)?
What you may gain
Fewer moderate or severe exacerbations over a year.
Fewer courses of rescue steroids or antibiotics.
Fewer urgent care visits and a lower chance of hospital admission.
Modest improvements in lung function in some people.
What you should not expect
A cure for COPD.
A quick fix in days.
A substitute for inhalers, pulmonary rehabilitation, and smoking cessation support.
Safety, side effects, and monitoring
Side effects differ by medicine, but common themes include injection-site reactions, headache, and occasional upper respiratory symptoms. Severe allergic reactions are uncommon but need urgent care if they occur.
Your team will monitor your flare-up pattern, infections, and response over months. If you do not see meaningful benefit, your clinician may recommend stopping and focusing on other strategies.
Frequently asked questions
1) Are biologics for COPD the same as biologics for asthma?
They can be the same medicines, but the target group in COPD is narrower as less patients have high eosinophils compared to asthma.
2) What eosinophil level is “high enough” for biologics for COPD?
It depends on the drug and local criteria. Some dupilumab COPD trials commonly used a threshold around 300 cells/µL. Other programmes may use different thresholds. Your clinician will interpret results across time and follow NICE guidance.
3) Will biologics help my breathlessness every day?
They can, but the main expected benefit is fewer flare-ups. Any day-to-day symptom improvement tends to be modest and varies by person.
4) Do I still need my inhalers if I start a biologic?
Yes. Biologics are add-on treatments. Your inhalers, rehab, and lifestyle measures remain the backbone of COPD care.
5) Are biologics safe if I get frequent chest infections?
This depends on your infection pattern, bronchiectasis risk, steroid exposure, and overall health. Your clinician will weigh risks and benefits for you as an individual.
GET IN TOUCH
Schedule a Visit with Dr Jose
For an assessment of your diagnosis and treatment.
Disclaimer: The information provided in this article is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment, and is not an advertisement for medical products. Always seek the advice of your healthcare provider with any questions you may have regarding a medical condition or treatment. Your healthcare professional can assess your individual circumstances. Consultation does not guarantee suitability for any specific treatment; all clinical decisions follow an individual assessment and shared decision-making
