Gefapixent for chronic cough is a potential option for adults whose cough carries on for months despite usual treatments and careful checks.
Chronic cough hypersensitivity means the cough reflex fires too easily to everyday triggers such as cold air, perfumes, talking, or laughter. People often feel an urge to cough that they cannot suppress, and this can disrupt sleep, work, relationships, and continence.
Doctors do not jump straight to new medicines. They first follow a structured pathway: look for warning signs, arrange appropriate tests, and treat common problems. They also offer behavioural cough control therapy, which teaches ways to calm the cough reflex and reduce throat irritation.
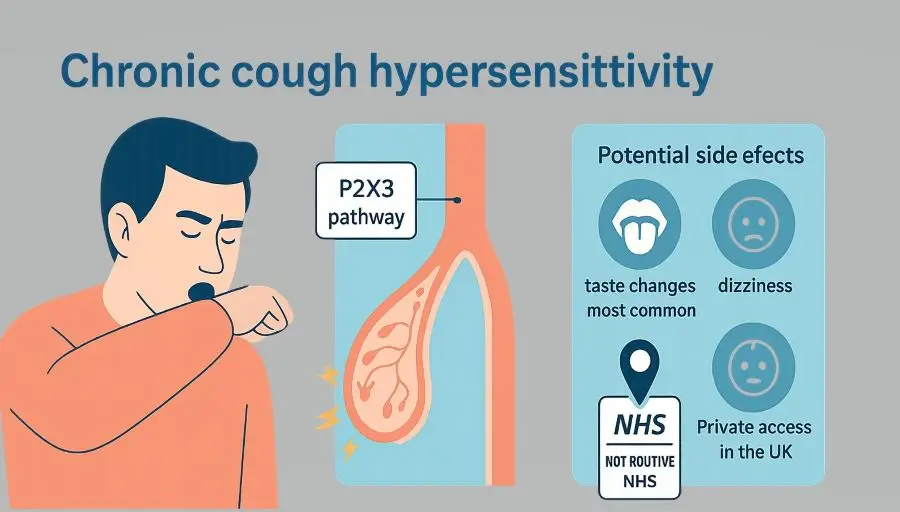
Gefapixent (brand name Lyfnua) fits in only when the cough remains refractory or unexplained after this pathway. It blocks P2X3 receptors on sensory nerves, which play a role in the over-sensitive cough reflex.
Quick take: what, who, status, dose
What it is: an oral antagonist of the P2X3 receptor that dampens sensory nerve signalling related to the urge-to-cough.
Who it’s for: adults with refractory or unexplained chronic cough once common causes have been assessed and optimised, and non-drug therapy has been offered.
UK status: licensed in Great Britain and the EU; not recommended by NICE for routine NHS funding. Access is typically through private prescribing where clinically appropriate.
Dose: 45 mg twice daily. Swallow tablets whole, with or without food. A lower dosing frequency may be used in severe renal impairment in people not on dialysis, as advised by the product information.
How gefapixent fits the biology of cough hypersensitivity
In inflamed or irritated airways, damaged cells can release ATP. ATP activates P2X3 receptors on vagal sensory fibres, driving an urge-to-cough and cough bouts. By blocking these receptors, gefapixent can reduce abnormal cough signalling in some people whose cough persists despite standard care. This makes it an option for refractory or unexplained chronic cough, rather than for acute cough or cough with a clear, untreated cause such as infection or uncontrolled asthma.
What the pivotal trials found
Two large phase 3 trials in adults with refractory or unexplained chronic cough compared gefapixent with placebo. At the licensed 45 mg twice-daily dose, gefapixent reduced objective 24-hour cough frequency versus placebo. The average benefit was modest but clinically meaningful for some individuals. The lower dose tested was not effective. Taste-related side effects were the most common reactions in the studies.
In practice, this means some people cough less often on treatment, but responses vary and side effects lead a proportion to stop therapy. Setting realistic expectations matters.
When to consider gefapixent for Chronic Cough
Gefapixent is considered only after a systematic pathway has confirmed chronic cough lasting 8 weeks or longer, ruled out red flags, and addressed treatable traits. Speech and language therapy-based cough control is very helpful and should be considered first because it has a favourable risk profile and can improve cough frequency. If cough remains refractory or unexplained and continues to impair daily life, a respiratory cough specialist may discuss gefapixent as an option.
People most likely to benefit include adults with a long history of burdensome cough who have tried and not responded to guideline-directed care, and who are prepared for a trade-off between potential cough reduction and a high likelihood of taste disturbance. Shared decision-making should set clear goals, such as fewer coughing fits, better sleep or improved continence during cough, and should include a plan to stop if benefit is absent or adverse effects are unacceptable.
When not to use it (or when to pause)
Do not use gefapixent for acute cough or when there is an untreated underlying cause. Do not use it if there is a known hypersensitivity to the active substance or excipients. It is not indicated in people under 18 years. Use during pregnancy and breast-feeding requires caution because human data are limited. In severe renal impairment, a reduced dosing frequency may be appropriate. If taste disturbance or other adverse effects are troublesome, reassess benefit versus harm and consider discontinuation.
Safety profile and what to monitor
Taste changes are the main side effect. Food or drink may taste different, weaker, or have no taste at all. Many people notice this, and it is the most common reason to stop the medicine. Taste usually returns after you stop, but everyone is different.
Other side effects can include feeling sick, diarrhoea, a dry mouth, dizziness, or a headache. These are usually mild to moderate.
Serious clashes with other medicines have not shown up so far, but your clinician will still check your current medicines and the latest guidance before you start.
You need to track whether the medicine helps without causing problems. You can keep a simple cough diary, fill in a short quality-of-life questionnaire, and ask family or friends to say what changes they notice. A review at 6 to 12 weeks will help determine if it is worth continuing the treatment.
Access in the UK: private versus NHS
Great Britain has granted a marketing authorisation for Lyfnua. NICE has not recommended it for routine NHS use, so NHS funding is not generally available. Prescribing therefore typically occurs in the private sector after specialist assessment, where doctors can issue private prescriptions when clinically appropriate and take responsibility for monitoring.
Private prescription costs vary; patients pay the full cost charged by their chosen pharmacy. Your NHS GP is not obliged to convert a private recommendation into an NHS prescription when it falls outside current NHS guidance.
For international context, the United States regulator has taken a different position on the available evidence, which explains differences in access and media coverage between countries. This does not change the UK licence.
Frequently asked questions
Is gefapixent a cure for chronic cough hypersensitivity?
No. Trials show a reduction in cough frequency on average at the licensed dose, but responses vary between individuals.
Can my NHS GP prescribe Gefapixent for Chronic Cough?
Because NICE has not recommended gefapixent for NHS use, routine NHS funding is not in place. Access currently occurs mainly via private prescribing after specialist review.
How long before I notice any change?
Trial assessments looked at outcomes at 12 and 24 weeks. Some people report earlier changes, but timing varies. A planned review around 6 to 12 weeks helps decide whether to continue.
How does it compare with non-drug therapy?
Behavioural cough control therapy remains an important, low-risk option and should be offered to suitable patients. Medicines and non-pharmacological strategies can be complementary in a comprehensive plan guided by a cough specialist.
What is the most common side-effect of Gefapixent?
Taste changes are the most common adverse effect. Food or drink may taste different, weaker, or have no taste at all. Taste usually returns after you stop, but everyone is different.
Conclusion
Gefapixent offers a licensed option for adults with refractory or unexplained chronic cough whose symptoms persist despite guideline-based care, but the average benefit is modest and taste-related adverse effects are common. In the UK, access is largely via private care because it is not currently recommended by NICE for NHS use. People considering treatment should do so after specialist assessment, with clear goals and an agreed plan to stop if it does not help.
This article is informational. It is not personalised medical advice, diagnosis, or an advertisement for any specific service or pharmacy. Readers should consult a qualified healthcare professional who can assess their individual circumstances.
GET IN TOUCH
Schedule a Visit with Dr Jose
For an assessment of your diagnosis and treatment.
Disclaimer: The information provided in this article is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment, and is not an advertisement for medical products. Always seek the advice of your healthcare provider with any questions you may have regarding a medical condition or treatment. Your healthcare professional can assess your individual circumstances. Consultation does not guarantee suitability for any specific treatment; all clinical decisions follow an individual assessment and shared decision-making
